How Long Does It Take for Clomid to Increase Testosterone
Abstract
Secondary hypogonadism is more common than primary gonadal failure and is seen in chronic and acute illnesses. Although testosterone has a role in erections, its importance in erectile dysfunction (ED) has been controversial. Hypogonadism produced by functional suppression of pituitary gonadotropins has been shown to correct with clomiphene citrate, but with a modest effect on sexual function. We wondered if longer treatment would produce improved results. A total of 178 men with secondary hypogonadism and ED received clomiphene citrate for 4 months. Sexual function improved in 75%, with no change in 25%, while significant increases in luteinizing hormone (P<0.001) and free testosterone (P<0.001) occurred in all patients. Multivariable analysis showed that responses decreased significantly with aging (P<0.05). Decreased responses also occurred in men with diabetes, hypertension, coronary artery disease, and multiple medication use. Since these conditions are more prevalent with aging, chronic disease may be a more important determinant of sexual dysfunction. Men with anxiety-related disorders responded better to normalization of testosterone. Assessment of androgen status should be accomplished in all men with ED. For those with lower than normal age-matched levels of testosterone treatment directed at normalizing testosterone with clomiphene citrate is a viable alternative to giving androgen supplements.
Introduction
Erectile dysfunction (ED) is highly prevalent and is estimated to affect nearly 30 million men.1 The incidence was reported to be 52% in men aged 40 to 70 y and more recently 31% in a younger group aged 18–59 y.2 The difference is reasonable as the incidence of ED increases with age, and aging is also accompanied by a decrease in circulating androgens.3 This is true whether the testosterone concentration is measured as the total fraction,4 the free fraction,3 or the bioavailable fraction.5,6 In addition, various illnesses lower testosterone levels independent of age.4,7 Acute illness has been long accepted to decrease testosterone and frequently pituitary luteinizing hormone levels.8,9,10 Chronic illnesses also may cause hypogonadism,3,11,12,13,14 which may be complicated by concomitant use of medications.15 Psychological as well as physical stressors commonly lead to secondary or hypogonadotropic hypogonadism16 and hypothalamic suppression of gonadotropin-releasing hormone is thought to be the cause.11,15,17
Hypogonadism has long been associated with sexual dysfunction.18,19,20,21,22 Hypogonadotropic hypogonadism is much more common than primary testicular failure.11,15,23,24 In our clinic, which has a bias toward endocrine referrals, 36% of approximately 1000 consultations for ED monitored over a 2-y period had hypogonadism.25 Of these, 6% had primary hypogonadism while 30% had secondary hypogonadism.
Measuring the free testosterone level is superior to measuring the total testosterone fraction,18 because the sex-hormone-binding globulin increases with age,3 which renders the total testosterone level falsely high in older men.
Success with testosterone replacement on sexual function has varied. Morales et al. 26 reported a positive response in over half of patients treated, but most studies reported successful treatment in a minority of patients.13,25 Korenman et al. 24 agreed and proposed that hypogonadotropic hypogonadism and impotence in older men are two separate issues that should be evaluated and treated separately.24 Our previous study illustrated that older men receiving clomiphene citrate stimulation can achieve higher testosterone levels.6 Clomiphene reversed the functional suppression of the central hypothalamic–pituitary axis, but did not achieve a good improvement in erectile function.11 When a secondary analysis was performed, the younger men, and those with fewer medical risk factors, seemed to achieve better sexual function, although the number of men was low and the duration of clomiphene stimulation was only 2 months. Burris et al. 27 followed hypogonadal men on testosterone replacement and reported a good effect after 3 months, with some continued improvement for several additional months. Conversely, it was also reported that it takes up to 6 weeks to notice clinical symptoms of androgen deficiency.28
We therefore wished to evaluate the treatment of functional hypogonadotropic hypogonadism with clomiphene citrate in a larger group of men than previously studied and for a longer period of time. We also wished to address if absence of a clinical response to normalizing testosterone was the result of age and/or comorbid medical factors.
Materials and methods
General patient population
In the Center for Sexual Function, 990 men who presented with sexual dysfunction during a 2-y period were evaluated with retrospective chart review. The clinical characteristics and medical risk factors of the population were previously reported.25 All men had testosterone and luteinizing hormone levels monitored, and 58 men (5.8%) had hypergonadotropic hypogonadism (primary gonadal failure), while 302 men (30.5%) had hypogonadotropic hypogonadism (ie, low testosterone and inappropriately low or normal luteinizing hormone levels). Of these 302 men, 272 (90.7%) had ED as part of their presenting complaint, defined as the inability to achieve or maintain an erection long enough to complete sexual intercourse satisfactorily in more than 50% of attempts.
Treatment with clomiphene citrate
Of the 272 men with hypogonadotropic hypogonadism and ED, 228 (83.8%) completed a 4-month course of clomiphene citrate, 50 mg orally on Monday, Wednesday, and Friday. Most of these men were married in a stable heterosexual relationship; the single men were in a steady relationship for at least 6 months. A home log was kept in which the couple recorded the number of sexual attempts and successes at intercourse. A successful response was defined as the ability to complete intercourse in more than 75% of attempts; a partial response was defined as successful intercourse in from 50 to 75% of attempts. The men who failed did not notice any change in their sexual activity. No men reported side effects caused by clomiphene citrate.
Hormone testing
Of the 228 men who began taking clomiphene, 173 (75.9%) men completed the 4-month course of treatment and had similar blood tests performed in the same laboratory, the Central Laboratory of Lahey Clinic. This population was the basis of the present study. Serum luteinizing hormone and free testosterone levels were drawn between 08:00 and 12:00. Of these 173 men with low baseline testosterone levels, 116 (or 67.1%) had two or more low pretreatment testosterone levels measured 1–3 months apart, which ruled out the possibility of random fluctuations or laboratory error.
The luteinizing hormone assay was performed with kits from Becton Dickinson Immunodiagnostics (Orangeburg, NY, USA) and the analog-free testosterone by direct radioimmunoassay with kits from Diagnostic Products Corporation (Los Angeles). The normal range for luteinizing hormone in men is 1–9 IU/l. The normal range for free testosterone is age related and is reported as 9.0 pg/ml or higher for age 70 y and older, over 11.0 pg/ml for ages 50–70 y, over 13.0 pg/ml for ages 40–50 y, and over 16.0 pg/ml for men under 40 y.
Statistical analyses
Patients were categorized as having complete, partial, or no response based on self-reported erectile function following clomiphene therapy. χ 2 analyses (or Fisher's exact tests, where appropriate) were used to determine significant associations between therapeutic response and comorbid clinical or psychosocial illnesses. When ordinal categorical variables were compared, the linear-by-linear test of association was used.
Paired t-tests were used to compare the mean hormone levels within each response group before and after treatment with clomiphene citrate. Paired t-tests were repeated to assess differences in age within response groups with selected comorbid conditions. Independent t-tests assuming equal variance were used to detect significant differences between response groups in the change (or delta) in luteinizing hormone and free testosterone levels following treatment.
Finally, multivariate analyses were conducted to determine the independent effects of patient characteristics and clinical risk factors on the likelihood of responding to clomiphene. The three response categories were collapsed into a binary variable, where 1 denoted any response to treatment (ie, positive or partial) and 0 denoted no response. Variables deemed clinically relevant or statistically significant in univariate analyses were selected for entry into multivariate models. Diagnostic analyses (eg, tests of multicollinearity and analysis of residuals) were conducted to ensure that variables were appropriately entered in the model and critical statistical assumptions were not violated. When odds ratios for scaled predictors were large, regression models were run with and without the parameter of interest and goodness-of-fit statistics were then compared. Adjusted odds ratios and 95% confidence intervals are reported. P values ≤0.05 indicate statistical significance, whereas P values >0.05 but <0.10 denote a trend. All P values are two-tailed. Statistical tests were conducted using SPSS Statistical Software for Windows, Version 10.0 (SPSS Inc., Chicago, 2001).
Results
Univariate analyses
The mean age of the 173 men was 54.3 y. A total of 84 men (48.6%) were under age 55 y (mean, 45.2 y), and 89 men (51.4%) were 56 y or older (mean, 62.1 y).
Figure 1 shows the response rate of the group as a whole. In all, 67 men (38.7%) had a positive response with a regular intercourse completion rate (>75%), 63 men (36.4%) had a partial response with an intercourse completion rate of 50–75%, and 43 men (24.8%) reported no change. Therefore, 75.1% noticed improvement in ED, with 38.7% returning to normal sexual function.
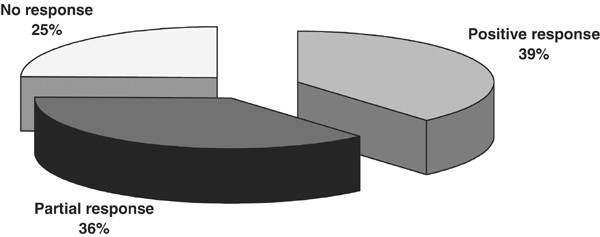
Effect if clomiphene citrate treatment in men with ED (N=173).
Full size image
Figure 2 and Figure 3 show that despite a disparity in clinical response, clomiphene stimulation significantly raised the blood levels of both luteinizing hormone and free testosterone in all groups. The serum luteinizing hormone level rose in the responders from 3.3 to 7.7 IU/l (P<0.001), from 4.2 to 7.7 IU/l (P<0.001 in the partial responders), and from 4.4 to 8.5 IU/l (P<0.001) in the nonresponders. The serum-free testosterone levels rose from 9.3 to 21.2 pg/ml in the responders, from 9.2 to 18.0 pg/ml in the partial responders, and from 9.8 to 17.6 pg/ml in the nonresponders (all significant where P<0.001).
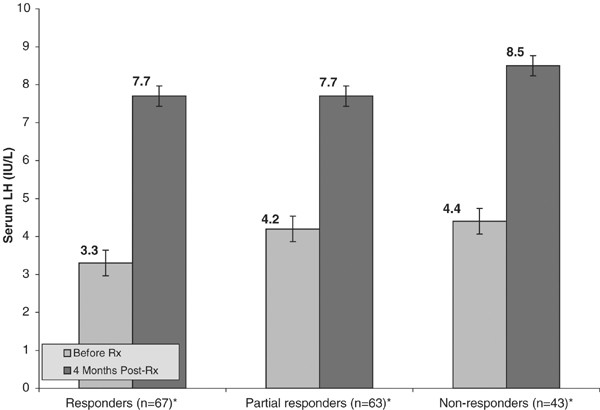
Effect of clomiphene citrate on LH (N=173). *Paired t-test reveal significant difference in means within-groups where P<0.01.
Full size image
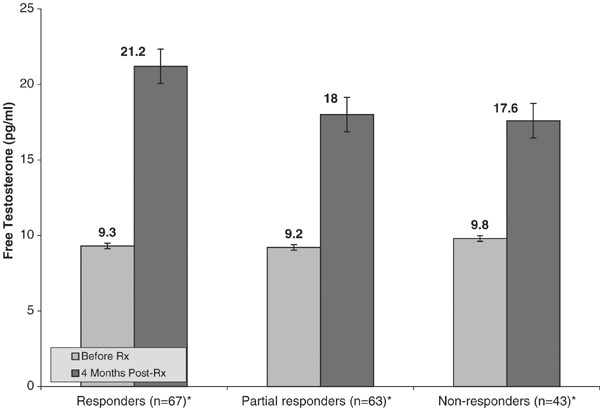
Effect of clomiphene citrate on free testosterone (N=173). *Paired t-test reveal significant difference in means within-groups where P<0.01.
Full size image
Figure 4 shows the response to clomiphene as a function of age. There was a higher percentage of responders (56.7%) to clomiphene stimulation in men 55 y and younger compared to 43.3% of the men over age 55 y. The partial responders were equally divided by age, 49.2% in the men under 55 y or younger and 50.8% in the men over 55 y. When categories were collapsed into any response vs no response, there were significantly more nonresponders in the age group over 55 y, 65.1 vs 34.9%, respectively (P<0.001). The association between increasing age and decreasing response to clomiphene was statistically significant (P=0.028).
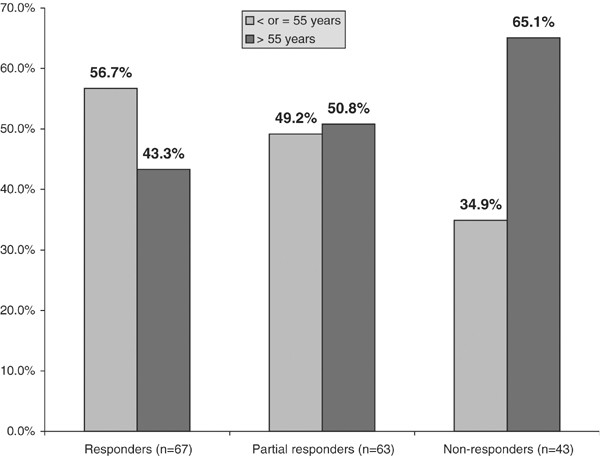
Age as a factor in the response to clomiphene citrate simulation (N=173). *Linear-by-linear association test indicates the likelihood of response to therapy decreases significantly as age increases (P=0.028).
Full size image
Table 1 shows the influence of comorbid medical risk factors on the effect of clomiphene-stimulated testosterone levels on erectile function. A higher number of partial responders and nonresponders were seen in patients with hypertension, diabetes mellitus, coronary artery disease (CAD), and those using multiple medications; however, not all of these differences achieved statistical significance. In these comorbidities, the number of men who responded partially to testosterone replacement was between 36.4 and 50.0%. In all, 36.4% of patients with diabetes were nonresponders, while 27.2% were responders (significant where P=0.02). In all, 50% of men taking multiple medications experienced partial response to clomiphene, while only 25.0% of these patients experienced complete response (P=0.02). Men with psychosocial comorbidities to their hypogonadism seemed to respond better to correction of the low testosterone, between 42.1 and 48.3%. This appeared to be true for various types of stress, whether because of endogenous chronic anxiety, performance anxiety, or work-related stress. It is interesting that significant performance anxiety was a greater problem for men who had a partial clomiphene response than for those with no response (44.7% partial response vs 13.2% nonresponse within this subgroup; P=0.05).
Full size table
Table 2 correlates the medical risk factors (diabetes, hypertension, CAD, and the use of multiple medications) with age. There were more responders who were 55 y and below with important medical risk factors of hypertension, diabetes, multiple medications, and CAD. Only the incidence of CAD however differed significantly by age (50.0% for younger men vs 19.0% for older men; P=0.02). Among partial responders, there was a higher percentage of younger men who had diabetes, CAD, and took multiple medications. Again, only the incidence of CAD differed significantly by age (40.0 vs 33.0%; P=0.05). In non-responders, there was a definite increase in the number of older men in all four of the major medical risk factors. Older nonresponders were significantly more likely to have hypertension than younger nonresponders (36.0 vs 7.0%, respectively; P=0.04). In the anxiety categories, mild performance anxiety failure was significantly higher in younger responders (57.0 vs 31.0%; P=0.03), while significant performance anxiety is greatest among older responders (60.0 vs 36.0%; P=0.001), younger partial responders (50.0 vs 30.0%; P=0.001), and younger nonresponders (14.0 vs 10.0%; P=0.04). Work stress and anxiety/depressive disorder failures are more prevalent among partial responders 55 y or younger (40.0 vs 18.0%; P=0.001 and 42.0 vs 33.0%; P=0.09, respectively).
Full size table
Multivariable analyses
Logistic regression analysis assessed the effects of relevant clinical variables while controlling for covariates. Since many variables were inter-related, a correlation matrix was created to assess problems of multicollinearity that would bias regression coefficients and inflate standard error estimates. Plots of residuals and goodness-of-fit statistics for models with and without selected variables were then analyzed. Variables with extremely low frequencies or high correlations with one another were ultimately excluded, because these decrease the predictive validity of the model. Table 3 shows the final logistic regression model and related parameter estimates.
Full size table
With respect to patient characteristics, age was the strongest predictor of partial or positive response to clomiphene (significant where P=0.05). When other clinical predictors were controlled for, patients aged 55 y and younger are almost 2.3 times as likely to respond to clomiphene treatment as patients 56 y and older. The absence of a venous leak, clinically manifested as early detumescence of sexually stimulated erections, was the second strongest predictor of therapeutic response. Patients who did not have venous leak symptoms, that is, early loss of erections, were about 63% less likely to respond to therapy than those who had a venous leak (P=0.04). In addition, patients taking multiple medications were 46% less likely to respond than patients taking fewer medications. However, this difference was not statistically significant (P=0.26).
Diabetes was the only clinical predictor of patient response that approached statistical significance. Patients with diabetes were 55% less likely than patients without diabetes to respond to clomiphene therapy (P=0.06). The narrow confidence interval around this estimate indicates that with a slightly higher sample size, the parameter would become significant at or below the 0.05 level. Contrary to published literature, cigarette smokers had virtually no difference in response compared with nonsmokers (OR, 1.02; P=0.98). However, this estimate may be attributable to the low number of smokers in the sample. Patients with hypertension or anxiety/depression were also less likely to respond to therapy, confirming results of univariate analyses (OR, 0.78 and 0.45, respectively; P=0.34 and 0.21). Finally, patients with CAD were 1.60 times as likely (60% more likely) than those without CAD to respond to therapy, although the result was not statistically significant (P=0.36).
The final three predictors illustrate the relations between previous clinical therapy for ED and clomiphene response. As a result of the wide variety of therapeutic options available to and used by these patients, three different categories were created: yohimbine therapy, Medicated Urethral System for Erection (MUSE) therapy, and other modalities (which included penile injections, vacuum pumps, or rings). Patients were only slightly more likely (11%) to respond to clomiphene if they had previously received yohimbine (OR, 1.11; P=0.80), but were somewhat more likely to respond if they had received MUSE therapy (OR, 1.60; P=0.27), although the result was not statistically significant. In contrast, the use of other therapeutic devices was a strong and significant predictor of response. Patients who had used alternative therapies were 3.9 times as likely to respond to clomiphene than those who had not received such therapy (P=0.002).
A secondary regression analysis was conducted using backward deletion of parameters to create a best-fitting model. Results confirmed the significance of the variables described previously. A multinomial logistic regression model was also run, using partial response, positive response, and no response categories as levels of the dependent variable. Parameters affecting therapeutic response in previous analyses did not differ significantly when the response was measured as three categories instead of two. Finally, tests for first-order interactions between age and clinical characteristics did not provide additional insights, and are therefore not included.
Discussion
Our data showed that clomiphene citrate can successfully stimulate the hypothalamus to cause increased testicular testosterone production. Stimulation with 50 mg of oral clomiphene three times weekly may be diagnostic as well as therapeutic. If the patient does not respond with at least a 75% increase in testosterone and a 100% increase in luteinizing hormone, further evaluation of the hypothalamic–pituitary area is warranted.15 All study patients met these criteria, yet only 39% of the men had a completely positive clinical response (ie, successful intercourse in at least 75% of attempts). The lack of complete response occurred mainly in men over 55 y of age. It was also seen in the presence of common chronic conditions such as diabetes mellitus, hypertension, CAD, and the use of multiple medications. The fact that these conditions were also more prevalent in the older age group seems to indicate that the lack of clinical response may be the result of comorbid medical factors than of age alone.
The fact that 39% of men responded completely suggests that testosterone is involved in erectile function. It has long been established that testosterone is required for libido in men, but there has been debate regarding the extent of its effect on erectile capacity and sexual satisfaction.29 Kwan et al 30 believed that sleep erections were testosterone dependent but that sexual erections were not, at least in the laboratory. A rough quantitation of the minimum amount of total testosterone necessary for nocturnal erections31 indicated that 200 ng/dl of total testosterone was adequate for maximal response. The positive effects of testosterone on nocturnal erections have also been shown by others, who reported a positive effect in coital attempts, and in orgasms as well.32 The dependence of the erectile response on testosterone has been suggested to be, at least in part, centrally mediated.33
At the biochemical level, androgens are necessary for the physiologic erectile response in the corpus cavernosum of the penis.34 Penile response to the intracavernosal injection of chemicals is better when there are adequate levels of testosterone.35 Androgens have also been shown to be necessary for the formation and activity of nitric oxide, held by most to be the most important intrapenile chemical transmitter needed for attaining of erections.36 Other actions have also been postulated, such as modulation of the intrapenile α-adrenergic mechanisms, which affects tumescence and detumescence.37,38,39
As previously mentioned, acute critical illness decreases testosterone levels, especially by suppressing the central hypothalamic-pituitary axis.15,17 The same is true of chronic illnesses. The effect may be mediated through cytokines produced in systemic diseases, including tumor necrosis factor.40 Decreasing testosterone production via a primary effect on steroidogenesis in the testicular Leydig cell may be seen in AIDS41 or by the effects of certain drugs such as ketoconazole42 or suramin.43 Chronic conditions such as diabetes mellitus,20 hypothyroidism,44 hemodialysis,45,46 or epilepsy47 may affect the central production of gonadotropins. Iron deposition in the pituitary may cause hypogonadotropic hypogonadism in beta-thalassemia,48 the effect of which was found to be separate from the production of diabetes in these patients. Some central suppression of testosterone production was even found during fasting in younger men.49 Some substances such as glucocorticoids50 and the Chinese herbal medicine Chansu51 may affect both the central hormonal axis and the testicles directly.
A large percentage of our patients had anxiety-related disorders; some had chronic anxiety, while others had performance anxiety, but the most prominent was work-related stress. Excess cortisol suppresses the central hypothalamic–pituitary axis, whether exogenous52 or endogenous.53,54,55 Both psychological and physical stress can cause increased endogenous cortisol production.56 Even the short-lived stress of preparing to skydive may increase cortisol production and suppress testosterone.57
Clomiphene stimulation did not elevate the testosterone level out of the normal range, but it remained in the middle of the normal range. Stimulation for 4 months produced levels similar to those in our previous study when the levels were measured after 2 months.11 This should be adequate for the desired clinical response. Wang et al 58 found that a positive response to testosterone did not improve further when testosterone levels increased from the low-normal to the high-normal range. Snyder et al 59 also found that increasing the testosterone level of normal, healthy males over the age of 65 to the level of young healthy males had no effect on energy, mental health, or sexual function.
It was suggested that a low testosterone level might only represent a random spurious value.60 We found this unlikely in our study, because 67% of our patients had two successive low testosterone values measured 2 weeks to 2 months apart before any therapeutic intervention. These low testosterone values also correlated with their presenting symptoms of libido and erectile difficulties. It is also doubtful that the positive responses were due to a placebo effect, because the results were similar to our previous placebo-controlled study11 and the home logs showed an increased intercourse rate that was verified by the partners.
Our results also agree with those of Jain,61 who showed a cause and effect relation between ED and low testosterone levels in a meta-analysis. The response rate was better in primary hypogonadsim than in secondary hypogonadism. We agree with Jain's explanation that men with primary testicular failure have a clearer presentation and fewer comorbidities than in secondary testicular failure, a factor that tends to confound the picture in the latter situation.
It does appear that many men may have functional suppression of the central component of their hypothalamic–pituitary–testicular axis resulting from a variety of acute and chronic medical conditions and multiple drug use. The same is true for various types of anxiety. The testosterone level generally can be increased well into the normal range with the use of clomiphene citrate three times weekly. A minority, 39%, of the men will clinically respond to this treatment totally and this group will not have to consider artificial means of correcting their sexual dysfunction, or treatment of their comorbid medical or psychological factors. We have found clinically that if the primary cause of the problem is corrected, clomiphene can occasionally be tapered and stopped, and the testosterone level will remain normal (unpublished observations). The total treatment rarely needs to be extended beyond 6 months. Quite frequently in men with ED and hypogonadism, correcting that the sexual problem may require additional methods of treatment. Nevertheless, correcting the testosterone deficit may have other beneficial effects. These may include increasing energy and well-being, as well as prevention of anemia or bone loss, depending on the severity of the hypogonadism. If patients cannot maintain their testosterone levels in the normal range after clomiphene is discontinued, permanent testosterone replacement with intramuscular injection, transdermal patches, or gels should be considered.
This study had empirical limitations. The relatively small number of patients who received treatment, combined with the low frequencies of many variables of interest, makes it difficult to create a robust logistic regression model. Smaller numbers increase residual variance, making it harder to assess the true magnitude of effect sizes with 95% confidence.
Clearly, an ideal study would match patients on key comorbid and clinical characteristics a priori (eg, by type and number of medications, age, past therapy for ED, and lifestyles), and would utilize a placebo-controlled, randomized design. However, we controlled for confounding variables a posteriori during regression analysis. We also conducted extensive diagnostic analyses before attempting the regression to ensure that variables were included appropriately. Finally, several competing models were compared and all results were similar, suggesting that the model exhibits a reasonable fit to the data.
These findings reinforce the recommendation to check testosterone levels in all men with ED. We also conclude that clomiphene stimulation may provide a reasonable option to correct hypogonadotropic hypogonadism, especially when it is functional in etiology and might be temporary in nature. An example of this scenario is a man with hypogonadism and sleep apnea (a very common combination), who may need testosterone therapy while starting a weight loss program and undergoing treatment with positive pressure nocturnal therapy. Owing to its low profile of side effects, clomiphene can be considered as a safe alternative form of short-term testosterone replacement. However, caution should be exercised in certain areas. We recently published our findings concerning testosterone and prostate-specific antigen (PSA) levels.62 The levels of PSA rose with all forms of testosterone treatment, including clomiphene citrate, from 0.2 to 1.2 ng/dl. Therefore, monitoring PSA levels before and after treatment is imperative, even with clomiphene stimulation. If the level of PSA is elevated before or after the clomiphene, the treatment should be stopped and the patient evaluated by a urologist. We recently had one young man who presented with the complaint of shimmering visual fields while on clomiphene (palleanopsia), which cleared after cessation of use. This has been reported in women using clomiphene during fertility treatment.63 Men should be warned about this possibility. Finally, this study should be replicated using a matched, randomized, prospective design in the general ED population, to ensure that results are free of ascertainment bias and to control for possible confounding variables during sample selection.
References
- 1
Feldman HA et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 151: 54–61.
CAS Article Google Scholar
- 2
Laumann EO, Paik A, Rosen RC . Sexual dysfunction in the United States. Prevalence and predictors. JAMA 1999; 281: 537–544.
CAS Article Google Scholar
- 3
Gray A, Feldman HA, McKinlay JB, Longcope C . Age, disease and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991; 73: 1016–1025.
CAS Article Google Scholar
- 4
Deslypere JP, Vermeulen A . Leydig cell function in normal men: effect of age, lifestyle, residence, diet, and activity. J Clin Endocrinol Metab 1984; 59: 955–962.
CAS Article Google Scholar
- 5
Nankin HR, Calkins JH . Decreased bioavailable testosterone in aging normal and impotent men. J Clin Endocrinol Metab 1986; 63: 1418–1420.
CAS Article Google Scholar
- 6
Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ . The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab 1987; 65: 1118–1126.
CAS Article Google Scholar
- 7
Blackman MR, Weintrau BD, Harman SM . Comparison of the effects of lung cancer, benign lung disease, and normal aging on pituitary–gonadal function in men. J Clin Endocrinol Metab 1988; 66: 88–95.
CAS Article Google Scholar
- 8
Woolf PD et al. Transient hypogonadotrophic hypogonadism after head trauma: effects on steroid precursors and correlation with sympathetic nervous system activity. Clin Endocrinol 1986; 25: 265–274.
CAS Article Google Scholar
- 9
Woolf PD, McDonald JV, Lee LA, Kelly M . Hypogonadism of critical illness. J Clin Endocrinol Metab 1985; 60: 444–450.
CAS Article Google Scholar
- 10
Van den Berghe G, de Zeghert F, Lauwers P, Veldhuls JD . Luteinizing hormone secretion and hypoandrogenaemia in critically ill men: effect of dopamine. Clin Endocrinol 1994; 41: 563–569.
CAS Article Google Scholar
- 11
Guay AT, Bansal S, Heatley GJ . Effect of raising endogenous testosterone levels in impotent men with secondary hypogonadism: double blind placebo-controlled trial with clomiphene citrate. J Clin Endocrinol Metab 1995; 80: 3546–3552.
CAS PubMed PubMed Central Google Scholar
- 12
ACCE Clinical Practice Guidelines for the evaluation and treatment of hypogonadism in adult male patients. Endo Pract 2: 198–211.
- 13
Bhasin S, Bremner WJ . 1997 Emerging issues in androgen replacement therapy. J Clin Endocrinol Metab 1996; 82: 3–8.
Google Scholar
- 14
Kosasih JB, Abbasi AA, Rudman D . Serum insulin-like growth factor-1 and serum testosterone status of elderly men in an inpatient rehabilitation unit. Am J Med Sci 1996; 311: 169–173.
CAS PubMed Google Scholar
- 15
Guay AT, Bansal S, Hodge MB . Possible hypothalamic impotence. Male counterpart to hypothalamic amenorrhea? Urology 1991; 38: 317–322.
CAS Article Google Scholar
- 16
Van den Bergeh G, de Zegher F, Bouillon R . Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab 1998; 83: 1827–1834.
Google Scholar
- 17
Giagulli VA, Kaufman JM, Vermeulen A . Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab 1994; 79: 997–1000.
CAS PubMed Google Scholar
- 18
Govier FE, McClure RD, Kramer-Levien D . Endocrine screening for sexual dysfunction using free testosterone determinations. J Urol 1996; 156: 405–408.
CAS Article Google Scholar
- 19
LoGiudice F, Trimarchi F, Benvenga S . High prevalence of hypogonadism in impotence (Abstract). 10th International Congress of Endocrinology, San Francisco, 1996.
- 20
Murray FT et al. Gonadal dysfunction in diabetic men with organic impotence. J Clin Endocrinol Metab 1987; 65: 127–135.
CAS Article Google Scholar
- 21
Spark RF, White RA, Connolly PB . Impotence is not always psychogenic. JAMA 1980; 243: 750–755.
CAS Article Google Scholar
- 22
Carroll JL, Ellis DJ, Bagley DH . Age-related changes in hormones in impotent men. Urology 1990; 36: 42–46.
CAS Article Google Scholar
- 23
Dobs AS, El-Deiry S, Wand G, Wiederkehr M . Central hypogonadism: distinguishing idiopathic low testosterone from pituitary tumors. Endo Pract 1998; 4: 355–359.
CAS Article Google Scholar
- 24
Koreman SG et al. Secondary hypogonadism in older men: its relation to impotence. J Clin Endocrinol Metab 1990; 71: 963–969.
Article Google Scholar
- 25
Guay AT, Velasquez E, Perez JB . Characterization of patients in a medical endocrine-based center for male sexual dysfunction. Endo Pract 1999; 5: 314–321.
CAS Article Google Scholar
- 26
Morales A, Johnston B, Heaton JPW, Lundie M . Testosterone supplementation for hypogonadal impotence: assessment of biochemical measures and therapeutic outcomes. J Urol 1997; 157: 849–854.
CAS Article Google Scholar
- 27
Burris AS et al. A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl 1992; 13: 297–304.
CAS PubMed Google Scholar
- 28
Bagatell CJ, Heiman JR, Rivier JE, Bremner WJ . Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. J Clin Endocrinol Metab 1994; 78: 711–716.
CAS PubMed Google Scholar
- 29
Schiavi RC, White D, Mandeli J, Levine AC . Effect of testosterone administration on sexual behavior and mood in men with erectile dysfunction. Arch Sex Behav 1997; 26: 231–241.
CAS Article Google Scholar
- 30
Kwan M et al. The nature of androgen action on male sexuality: a combined laboratory-self-report study on hypogonadal men. J Clin Endocrinol Metab 1983; 57: 557–562.
CAS Article Google Scholar
- 31
Granata ARM et al. Relationship between sleep-related erections and testosterone levels in men. J Androl 1997; 18: 522–527.
CAS PubMed Google Scholar
- 32
Davidson JM, Camargo CA, Smith ER . Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab 1979; 48: 955–958.
CAS Article Google Scholar
- 33
Heaton JPW, Varrin SJ . Effects of castration and exogenous testosterone supplementation in an animal model of penile erection. J Urol 1994; 151: 797–800.
CAS Article Google Scholar
- 34
Mills TM, Reilly CM, Lewis RW . Androgens and penile erection: a review. J Androl 1996; 17: 633–638.
CAS PubMed Google Scholar
- 35
Bivalacqua TJ et al. The influence of castration on pharmacologically induced penile erection in the cat. J Androl 1998; 19: 551–557.
CAS PubMed Google Scholar
- 36
Reilly CM, Stopper VS, Mills TM . Androgens modulate the α-adrenergic responsiveness of vascular smooth muscle in the corpus cavernosum. J Androl 1997; 18: 26–31.
CAS PubMed Google Scholar
- 37
Reilly CM, Lewis RW, Stopper VS, Mills TM . Androgenic maintenance of the rat erectile response via a non-nitric-oxide-dependent pathway. J Androl 1997; 18: 588–594.
CAS Google Scholar
- 38
Traish AM, Kim NN, Goldstein I, Moreland RB . Alpha-adrenergic receptors in the penis: identification, characterization, and physiological function. J Androl 1999; 20: 671–682.
CAS Google Scholar
- 39
Aversa A et al. Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin Endocrinol 2000; 53: 517–522.
CAS Article Google Scholar
- 40
van der Poli T, Romijn JA, Endert E, Sauerwein HP . Effects of tumor necrosis factor on the hypothalmic–pituitary–testicular axis in healthy men. Metabolism 1993; 42: 303–307.
Article Google Scholar
- 41
Salchian B et al. Testicular pathologic changes and the pituitary–testicular axis during human immunodeficiency virus infection. Endo Pract 1999; 5: 1–9.
Article Google Scholar
- 42
Zwart AD, Iranmanesh A, Veldhuis JD . Disparate serum free testosterone concentrations and degrees of hypothalamo–pituitary–luteinizing hormone suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab 1997; 82: 2062–2069.
CAS PubMed Google Scholar
- 43
Danesi R et al. Clinical and experimental evidence of inhibition of testosterone production by suramin. J Clin Endocrinol Metab 1996; 81: 2238–2246.
CAS PubMed Google Scholar
- 44
Donnelly P, White C . Testicular dysfunction in men with primary hypothyroidism; reversal of hypogonadotrophic hypogonadism with replacement thyroxine. Clin Endocrinol 2000; 52: 197–201.
CAS Article Google Scholar
- 45
Mitchell R et al. Less acidic forms of luteinizing hormone are associated with lower testosterone secretion in men on haemodialysis treatment. Clin Endocrinol 1994; 1: 65–73.
Article Google Scholar
- 46
Hayami S, Sasagawa I, Nakada T . Influence of sex hormones on prostate volume in men on hemodialysis. J Androl 2000; 21: 258–261.
CAS PubMed Google Scholar
- 47
Murialdo G et al. Sex hormones and pituitary function in male epileptic patients with altered or normal sexuality. Epilepsia 1995; 36: 360–365.
CAS Article Google Scholar
- 48
Wang C, Tso SC, Todd D . Hypogonadotropic hypogonadism in severe β-thalassemia: effect of chelation and pulsatile gonadotropin-releasing hormone therapy. J Clin Endocrinol Metab 1989; 68: 511–516.
CAS Article Google Scholar
- 49
Bergendahl M et al. Fasting suppresses pulsatile luteinizing hormone (LH) secretion and enhances orderliness of LH release in young but not older men. J Clin Endocrinol Metab 1998; 83: 1967–1975.
CAS PubMed Google Scholar
- 50
Odell WD . Testosterone treatment of men treated with glucocorticoids. Arch Intern Med 1996; 156: 1133–1134.
CAS Article Google Scholar
- 51
Wang S-W et al. Effects of methanol extract of Chansu on hypothalamic–pituitary–testis function in rats. Metabolism 1998; 47: 1211–1216.
CAS Article Google Scholar
- 52
Reid IR, Wattie DJ, Evans MC, Stapleton JP . Testosterone therapy in glucocorticoid-treated men. Arch Intern Med 1996; 156: 1173–1176.
CAS Article Google Scholar
- 53
Rosmond R, Dallman MF, Bjorntorp P . Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 1998; 83: 1853–1859
CAS PubMed Google Scholar
- 54
Veldhuis JD . Recent insights into neuroendocrine mechanisms of aging of the human male hypothalmic–pituitary–gonadal axis. J Androl 20: 1–17.
- 55
Hardy MP, Ganjam VK . 1997 Stress, 11β-HSD, and Leydig cell function. J Androl 1999; 18: 475–479.
Google Scholar
- 56
Singh A et al. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J Clin Endocrinol Metab 1999; 84: 1944–1948.
CAS PubMed Google Scholar
- 57
Chatterton RT Jr, Vogelsong KM, Lu Y-C, Hudgens GA . Hormonal responses to psychological stress in men preparing for skydiving. J Clin Endocrinol Metab 1997; 82: 2503–2509.
CAS PubMed Google Scholar
- 58
Wang C et al. Testosterone replacement therapy improves mood in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab 1996; 81: 3578–3583.
CAS PubMed PubMed Central Google Scholar
- 59
Synder PJ et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999; 84: 1966–1972.
Google Scholar
- 60
Spratt DI et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol 1988; 17: E658–E666.
Google Scholar
- 61
Jain P, Rademaker AW, McVary KT . Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164: 371–375.
CAS Article Google Scholar
- 62
Guay AT, Perez JB, Fitaihi WA, Vereb M . Testosterone treatment in hypogonal men: prostate-specific antigen level and risk of prostate cancer. Endo Pract 2000; 6: 132–138.
CAS Article Google Scholar
- 63
Purvin VA . Visual disturbance secondary to clomiphene citrate. Arch Ophthalmol 1995; 113: 482–484.
CAS Article Google Scholar
Download references
Acknowledgements
We thank Ms Gail Macey for her work as research coordinator, and Ms Polly Zorolow and Ms Lynda Charters for their editorial assistance.
Rights and permissions
About this article
Cite this article
Guay, A., Jacobson, J., Perez, J. et al. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit?. Int J Impot Res 15, 156–165 (2003). https://doi.org/10.1038/sj.ijir.3900981
Download citation
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/sj.ijir.3900981
Keywords
- clomiphene citrate
- erectile dysfunction
- hypogonadotropic hypogonadism
Further reading
-
Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study
- Andressa Heimbecher Soares
- Nidia Celeste Horie
- Cintia Cercato
International Journal of Obesity (2018)
-
Sexual dysfunction and male infertility
- Francesco Lotti
- Mario Maggi
Nature Reviews Urology (2018)
-
Mannelijk hypogonadisme, een update
- Hermanus H. J. Leliefeld
- Gert R. Dohle
Tijdschrift voor Urologie (2018)
-
The safety and efficacy of clomiphene citrate in hypoandrogenic and subfertile men
- D P Patel
- W O Brant
- J M Hotaling
International Journal of Impotence Research (2015)
-
Alternative Treatment Modalities for the Hypogonadal Patient
- Landon W. Trost
- Mohit Khera
Current Urology Reports (2014)
How Long Does It Take for Clomid to Increase Testosterone
Source: https://www.nature.com/articles/3900981